Our Mission
At Anwita Biosciences, our mission is to precisely reprogram immunity and tumor biology to deliver durable benefits for patients. Building on our clinical-stage targeted cytokine AWT020 (anti-PD-1-IL-2 fusion protein), we are advancing a diversified pipeline that includes next-generation antibody-drug conjugates (ADCs) with novel mechanisms, multi-specific T-cell engagers, and program targeting the drivers of metabolic dysfunction in cancer cachexia. Our translation-first approach combines deep biological understanding with rational engineering for selectivity and safety, enabling the rapid advancement of innovative therapies into the clinic.
Science
Technology
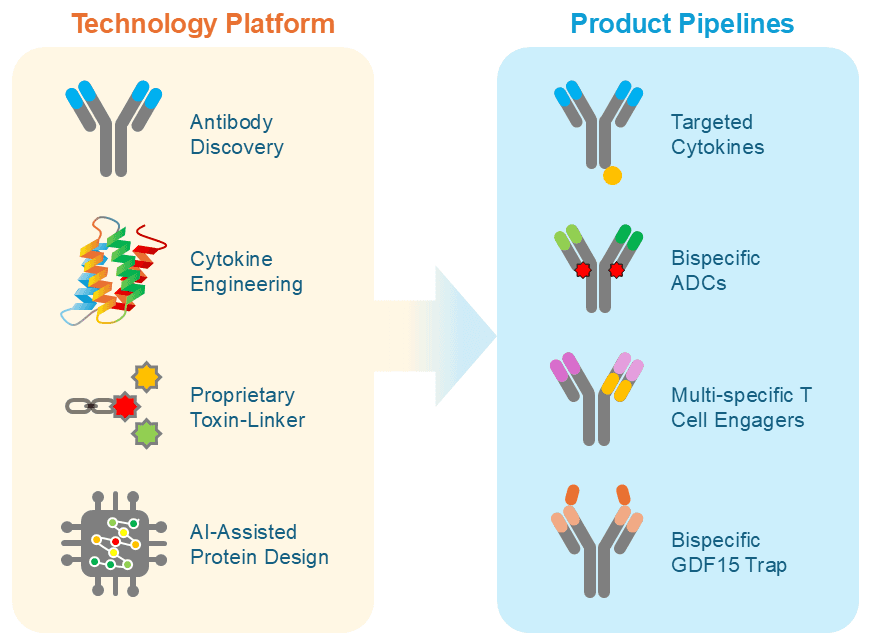
Anwita’s technology platform integrates antibody discovery, cytokine engineering, proprietary toxin-linker technologies, and AI-assisted protein design. By combining structure- and sequence-based modeling with experimental data, we precisely tune key molecular features ̶ target affinity, receptor bias, safety, TME-dependent payload release, and developability ̶ while accelerating the path from design concept to clinic.
The technology platform supports a diversified pipeline including:
- Targeted cytokine fusion proteins led by clinical asset AWT020 (anti-PD-1–IL-2) designed to direct immune activation locally within the tumor microenvironment.
- Bispecific ADCs with novel mechanisms of action.
- Multi-specific T-cell engagers that address antigen heterogeneity and immune resistance.
- A bispecific GDF15 trap in development for the treatment of cancer cachexia.
Publications
- Jermaine C. et. al. First-in-human study of AWT020, a bifunctional anti-PD-1/IL-2 fusion protein, in patients with advanced cancer. https://doi.org/10.1200/JCO.2025.43.16_suppl.e14500
- Jiang H. et al. NK cells limit the synergistic anti-tumor effect of PD-1 inhibition and βγ-biased IL-2, International Journal of Biological Macromolecules. https://doi.org/10.1016/j.ijbiomac.2025.147437
- Ye F. et al. AWT020: a novel fusion protein harnessing PD-1 blockade and selective IL-2 Cis-activation for enhanced anti-tumor immunity and diminished toxicity. https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1537466/full
- Ye F. et al. Synergistic enhancement of immunogenic cell death: Combining AWT020 with antibody-drug conjugates for superior anti-tumor activity. https://doi.org/10.1158/1538-7445.AM2025-4774
- Ye F. et al. A Safe and Highly Potent αPD1-IL2 Fusion (AWT020) that Decouples the Efficacy and Toxicity of IL-2 Therapy. https://anwitabio.com/events/2022/sitc/poster/pd1-il2.pdf
- Huang Z. et al. A highly potent anti-LAG-3-IL-2C that selectively targets tumor specific CD8+ T cells. https://anwitabio.com/events/2022/sitc/poster/lag3-il2.pdf
- Cheng X. et al. Exenokine-2: a half-life extended no-α-IL-2 with improved preclinical pharmacological properties supports first-in-human clinical development. https://anwitabio.com/events/2022/sitc/poster/exn-2.pdf
- Ye F. et al. A safe and highly potent anti-PD1-IL-2C fusion that decouples efficacy and toxicity of IL-2 therapy. https://anwitabio.com/aacr-poster.png
- Liu H. et al. An engineered IL-21 with half-life extension enhances anti-tumor immunity as a monotherapy or in combination with PD-1 or TIGIT blockade. Int Immunopharmacol. 2021 Dec;101(Pt A):108307. doi: 10.1016/j.intimp.2021.108307. Epub 2021 Nov 1. PMID: 34735918. https://pubmed.ncbi.nlm.nih.gov/34735918/
- Wu S. et al. The Half-Life-Extended IL21 can Be Combined With Multiple Checkpoint Inhibitors for Tumor Immunotherapy. Front Cell Dev Biol. 2021 Nov 15;9:779865. doi: 10.3389/fcell.2021.779865. PMID: 34869384; PMCID: PMC8634682. https://pubmed.ncbi.nlm.nih.gov/34735918/
Pipeline
Program
MOA
Patient Population
Preclinical
Lead ID
Lead OP
IND Enabling
Phase 1
Targeted Cytokines
AWT020
PD-1 and IL-2
NSCLC, GI and gynecological cancers
LAG-3 and IL-2
Melanoma combined with aPD-1; CART
Bispecific ADCs
EGFR and HER3
NSCLC; CRC
VEGF and PD-L1
Solid tumors
T-cell Engagers
BCMA, CD19, CD3
Hematological autoimmune disease
Multiple Targets
Solid tumors
Cancer Cachexia
GDF15 and IL-6
Solid tumors
Click or tap on any Program to read more about that project.
Our Leadership
News
Date: October 21st, 2025
Anwita Biosciences Receives FDA Orphan Drug Designation for AWT020 in Thymic Epithelial Tumors
Date: October 5th, 2022
Anwita Biosciences, Inc. Announces Multiple Poster Presentations Showcasing Its Three Cytokine-Based Therapeutics at 37th Society for Immunotherapy of Cancer (SITC) 2022 Annual Meeting
Date: September 21st, 2022
Anwita Biosciences Announced Today Dosing of First Patient in Its Phase I Clinical Trial of JS014, a Half-life Extended IL-21, for the Treatment of Solid Tumors
Date: March 15th, 2022
Anwita Biosciences, Inc. announces presentation on Mableukine-2PD1 at the 2022 annual American Association of Cancer Research (AACR) meeting.
Date: October 29th, 2021
Anwita Biosciences, Inc. Announces Initiation of first-in-human Phase 1 clinical trial of Exenokine-21 for monotherapy and combination, earns $2.5 million milestone for IND acceptance from Partner Shanghai Junshi Biosciences
Date: June 28th, 2021
Anwita Biosciences, Inc. Completes $18.5 Million Series B Financing to Advance Its Improved Cytokines (Exenokines) And Tumor Targeting Antibody Drug Conjugates
Date: September 30th, 2020
Anwita Biosciences and Shanghai Junshi Biosciences entered into a new collaboration in which Junshi was granted the exclusive rights to Anwita's Exenokine-2, an improved IL-2 variant with extended half-life, in the Greater China territories.
Date: June 24th, 2019
Strategic collaboration between Anwita Biosciences and Shanghai Junshi Biosciences whereby Junshi was granted the exclusive rights to develop and commercialize Anwita's Exenokine-21 program in the Greater China territories.
Careers
Openings
Scientist
Contribute to experimental planning, implementation, and data analysis; Independently design plasmid maps, primers and cloning strategies for antibodies and fusion proteins; Use ExpiCHO transient transfection and expression system to produce new proteins; Purify protein from ExpiCHO expression system and use analytical instrument including HPLC, AKTA to characterize protein quantity, quality and purity; Design mutations to change certain property of target protein, including receptor binding, stability and productivity; Use Octet Red96 instrument to perform binding assays and measure the dissociation constant; Perform cell based binding and functional screening assays to ID target specific antibody binders; Generate stable expression cell lines for cell based assays using pAS-puro vector and puromycin selection; Support antibody discovery team and perform phage display library construction and screening; Design and perform cell based assays, including cell surface staining, signaling assay and flow cytometry; Prepare reports and present in weekly group meeting.
- Master's Degree (or foreign equivalent) in Molecular Biology, Biomedical Engineering, or related field
- 2 years of experience in a research position in a biotechnology company. Position may require experience with:
- molecular cloning
- protein purification and characterization
- cell culture
Benefits
- Competitive compensation
- Stock options at all levels
- 401(K) plan
- Official holidays
- Generous paid time off (PTO)
- Sick days
- Medical plan
- Dental plan
- Visional plan
- Life insurance
Contact
Company Address:
300 Industrial Road,
San Carlos, CA 94070
Phone: (650) 600-9828
Email: contact@anwitabio.com
